Be a part of us as we look back again at one of the most-examine Health care Packaging stories of 2021. At #two: New tech developments have bolstered using BFS in aseptic processing, including temperature Management and needle addition for pre-filled syringes.
This free of charge, Website-based mostly application simplifies products choice and also the overall process that surrounds cable and pipe transits.
Lighting situations: Guarantee proper lighting circumstances inside the inspection space to improve visibility and detect any likely defects or contaminants.
For developing biologics, both GMP and biosafety needs have to be regarded as, and an interdisciplinary engineering tactic must be employed for right integration in the gear within the cleanroom and safe operation of the facility, claims Trapl.
Biological drug products and solutions, for example proteins or monoclonal antibodies, are predominately packaged into vials or prefilled syringes for intravenous or subcutaneous administration. Nonetheless, some biological drug products and solutions have to be administered by choice routes, like pulmonary shipping in the form of a mist using a nebulizer. In such a circumstance, making use of plastic ampuls as the principal drug container provides numerous pros above vials or syringes. Plastic ampuls are effortless, basic to utilize, are unbreakable, and boy or girl-pleasant.
A single-sided installation procedure allows reduce accessibility difficulties and enhances installation flexibility when determining from what facet in the deck or bulkhead to set up the pipe seal.
You'll find a number of substrates Employed in the look of packages with intent to deliver copyright and tamper obvious features ranging from litho paper, polystyrenes, destructive vinyl's, acetate movies artificial paper and coatings etcetera., There are lots of ways of incorporating covert markers inside of a substrate, for example seen or UV fluorescing fibers, or chemical reagents in carton board or paper. Watermarks may be embedded in leaflet paper, or metallic threads interwoven in the base materials, potentially like an overt optically variable products (OVD) element.
This ground breaking technique will involve the simultaneous development, filling, and sealing of containers, enabling for the production of sterile packaging in a very effective and controlled way.
Skinny micronic threads are launched inside the substrates both at the label stock earning stage or These are individually designed into two levels of paper laminated jointly. The threads will also be delicate to UV light-weight which can glow under UV gentle. e.g., forex notes.
The technology consists of the generation of the random, pseudo random code in a sequential way from the technology company entered into their or the customers information foundation for later verification. These codes are provided to consumers who consequently can utilize them in different ways. These codes may be more info printed about the labels and then affixed to the product or service or can be employed within a covert way on a pack.
On this unique rotary BFS device, the 15 or twenty sets of molds shift in sync Using the parison. As one particular set of molds moves down away from the filling needle, The underside of the subsequent set of molds seals the container, whilst the best of your mould kinds the next container.
Various polymers can be Employed in the method, reduced and substantial-density polyethylene and polypropylene remaining the preferred. The innate capacity to type the container/closure all through the particular aseptic packaging course of action permits personalized style of your container to satisfy the precise demands of the applying.
Visible inspection poses special challenges in Blow-Fill-Seal Technology mainly because of the constrained transparency of polymer containers in comparison with common glass vials. The partially transparent mother nature of your containers makes it tricky to visually inspect the contents for any probable defects or contaminants.
The benefits designed in the inhalation drug sector can be straight applied to unitdose non-preserved vaccines. Because more info the BFS program might be tailored for particular person purposes the inhalation vial is often adjusted to sort containers from 0.one mL to 3 mL with closures designed for intranasal, injection and oral dose functionality.
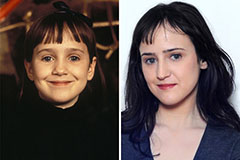 Mara Wilson Then & Now!
Mara Wilson Then & Now! Anthony Michael Hall Then & Now!
Anthony Michael Hall Then & Now! Marques Houston Then & Now!
Marques Houston Then & Now! Seth Green Then & Now!
Seth Green Then & Now! Loni Anderson Then & Now!
Loni Anderson Then & Now!